Luteal Phase Defects: A Possible Relationship between Short Hyperthermic Phase and Sporadic Sexual Behavior in Women

Winnifred B. Cutler, Ph.D., Celso Ramon Garcia, M.D., and Abba M. Krieger.
Hormones and Behavior 13, 214-218 (1979).
© Copyright 1979. Academic Press, Inc.
Abstract:
Sporadic (less than regular weekly) coital activity is associated with a particular type of infertility – the short hyperthermic (luteal) phase. This preliminary report is the first suggestion that a reduced frequency of human sexual activity might, itself, be associated with subfertile reproductive function.
Intro:
The endocrine correlates of luteal phase deficits, in infertility, have been well described. After ovulation, there is a defect in steroid secretion (luteual insufficiency) which is reflected in subnormal or early peaking of postovulatory plasma progesterone(Abraham, Odell, Swerdloff, and Hopper 1972: Abraham, Margulis & Marshall, 1974: Sherman and Korenman, 1974a, 1974b; Jones, 1976: Del Pozo, Wyss, Tolis, Alcaniz, Campana and Naftolin, 1979) with occassional high levels preovulatory (Dodson, MacNaughton, and Coutts, 1975). Suggested causes for the defect include: interference of corpus luteum function by prolactin, inadequate stimulation of granulosa layer by FSH, improper induction of luteinization by the midcycle LH surge (Jones, 1973: Taubert, 1978) and insufficient postovulatory maintenance levels of LH (Vande Wiele, Bonumil, Dyrenfurth, Ferin, Jewelewicz, Warren, Rizkallah, and Mikhail, 1970).
Cycles with ovulation-menstruation intervals of less than 12 days whether measured by repeated plasma progesterone throughout the cycles or by basal body temperature patterns reflect luteal phase defects (Corenblum, Pairaudeau, and Shewchuk, 1976; Wilks, Hodgen, and Ross, 1977; Tabuert, 1978; Del Pozo et al., 1979). About 20% of infertility patients present with the defect (Abraham et al., 1974)
Sexual behavior and reproductive deficits are associated. Delayed age at first coitus was associated with a high incidence of primary infertility (Cutler, Garcia, and Krieger, 1979) and childlessness (Masnick and McFalls, 1978): and sporadic (less than weekly) coital activity throughout the menstrual cycles, was associated with extremely short or long (<26 or >33 days) cycles (Cutler, Garcia, and Krieger, 1980).Since sporadic sexual behavior and luteal phase defects were both related to menstrual cycle length anomalies, it seemed appropriate to question whether sporadic sexual behavior might be related to luteal phase defects.
METHODS
A review of the active files of the infertility-gynecological practice of one author (CRG) produced a sample2 of 192 patient histories that contained prospective3 basal body temperature charts. A period of hyperthermia in the biphasic temperature graph was used to define the postovulatory (luteal phase) length by following classic4 procedures (Garcia and Rosenfeld, 1977). Careful scrutiny of the files yielded 60 usable records which met the following minimal criteria: legible data for at least one menstrual cycle defined by the recording of its two catamenial limits; coital data legibly recorded on a chart; attributable date of the recordings which then permitted confirmation that no drugs were ingested for the past 3 months, and absence of any cervical invasion during the cycle studied. Only the first cycle which met the above listed criteria was entered in order to avoid statistical problems of dependency and different amounts of information per subject.
These 60 patients presented a variety of infertility diagnoses such as tubal occlusion, pelvic adhesions, polycystic ovaries, habitual abortion, and ectopic pregnancy. They ranged in age from 20 to 41 years. All were attempting conception and most were nulliparous. Each patient’s coital frequency was defined as Weekly or Sporadic. Weekly classification required at least one coital event each nonmenstruating week of the cycle; Sporadic classification defined those women who abstained >7 continuous nonmenstruating days.
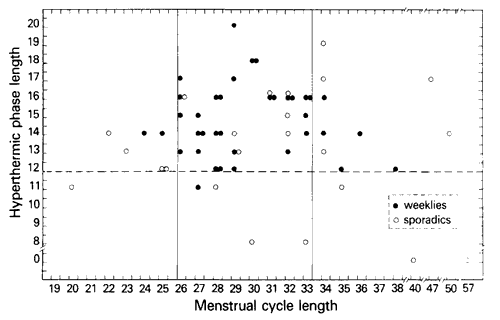
Fig. 1. Coital activity, cycle length, hyperthermal phase length 60 infertility patients by basal body temperature data. (Infertility patients N = 60.)
RESULTS
Figure 1 shows the lengths of the menstrual cycle (X axis) and hyperthermal phase (Y axis) of each of the 60 patients. It is noted that there are 8 women with deficient hyperthermic phases – i.e., less than 12 days duration. Seven of these eight show sporadic sexual behavior (See Table 1). In contrast, only 16 out of the 52 women with adequate hyperthermic phase lengths had sporadic sexual behavior. This implies that inadequate hyperthermic phase length and sporadic coital frequency are associated (P < 0.005, Fisher exact probability test) (Dixon and Massey, 1969). In addition, careful scrutiny of each of the basal body temperature charts showed that women who did miss a week of coitus (and thereby titled Sporadic) almost always did so during the luteal phase rather than the preovulatory follicular phase.
Table 1.
Coital Frequency vs Hyperthermal Phase Basal Body Temperature Dataa
Coital Frequency |
|||
Weekly |
Sporadic |
||
| Hyperthermic Phase |
|||
>12 days |
36 |
16 |
52 |
< 12 days |
1 |
7 |
8 |
37 |
23 |
60 |
|
a Infertility patients. N=60.
DISCUSSION
Seven of the eight women presenting short hyperthermic phases also showed sporadic (less than weekly) coital activity. By using only basal body temperature graphs to identify such defects (as this study did), one might not retrieve the total actual corpus luteum inadequacies which are present (Jones, 1976: Rosenfeld and Garcia, 1976).
While the luteal phase defect has been described and characterized by various criteria, there has not been a complete unanimity of precisely what is the most effective way of assessing and confirming its presence. Some of the techniques, i.e., the combined endometrial biopsy and plasma progesterone evaluation, are more encompassing than others (Rosenfeld and Garcia, 1976). In some instances the techniques themselves (endometrial biopsy) may have an influence on the cycle. Accordingly, in this presentation only the hyperthermic criteria was addressed in order to use the simplest and probably the least invasive method for the specific subject being evaluated.
These findings, although quite preliminary, are presented to suggest that other investigators further explore whether sporadic sexual activity might be one factor, in the cluster of factors, which comprise the luteal phase defect.
Since all the patients were trying to conceive and all were made aware of the need for follicular phase insemination; one might have predicted that those couples who had an abstinent week (sporadic classification) would have it after ovulation, i.e., in the luteal phase. Such was the case. The raw data showed that that among the sporadically active women, at least 1 week during the luteal phase contained no intercourse.
An alternative possibility is that luteal phase deficiencies with their concomitant reduced progesterone levels might produce a loss of sexual interest. There is, however, as yet, no clear evidence for an inhibitory role of reduced progesterone on the sexual behavior of women. These data might, therefore, suggest that luteal phase sexual activity serves some useful function in maintaining the corpus luteum.
In contrast with animals which lack a distinct luteal phase (e.g., the rat) humans engage in coitus throughout the entire cycle. One could speculate that coital activity throughout the cycle optimizes fertility in women. One intriguing possibility – that a neuroendocrine response to sexual stimulation provides the hormonal influence necessary for supporting the corpus luteum – might well be supported by future studies.
ACKNOWLEDGMENTS
We thank N.T. Adler for his contributions to this report and J. Levy for her original support.
Footnotes
2. Data are alphabetically obtained, but in any given week of sampling, some records are unavailable for review due to administrative patient care requirements and the techniques used to assure confidentiality.
3. It is standard practice of this infertility service to ask new patients to maintain, on specially provided forms, daily records of basal body temperature begins. It is our impression that people are aware of the importance of accurate reporting when they have a problem and are seeking a solution. Moreover, every effort is made to ensure accurate reporting by the subject.
4. After drawing a line representing the mode of the preovulatory phase temperatures and a line representing the mode of the postovulatory phase temperature recordings the last low point before the rise which is sustained is taken as the thermal nadir reference point - suggesting the probable time of ovulation.References
Abraham, G.E., Odell, W.D., Swerdloff, R.S., and Hopper, K. (1972). Simultaneous radioimmunoassay of plasma FSH, LH, progesterone, 17-hydroxyprogesterone and estradiol 17B during the menstrual cycle. J. Clin. Endocrinol. Metab. 34, 312-318.
Abraham, G.E., Maroulis, G.B., Marshall, J.R. (1974). Evaluation of ovulation and corpus luteum function using measurements of plasma progesterone Obstet. Gynecol. 44, 522-525.
Corenblum, B., Pairaudeau, N., and Shewchuk, A.B. (1976). Prolactin hypersecretion and short luteal phase defects. Obstet. Gynecol. 47, 486-488
Cutler, W.B., Garcia, C.G., and Krieger, A.M. (1979). Infertility and age at first coitus: a possible association. J. Biosoc. Sci. 11, 425-432.
Cutler, W.B. Garcia, C.G. and Krieger, A.M. (1979) Sexual behavior frequency and menstrual cycle length in mature premenopausal women. Psychoneuroendocrinology, 4, 297-309.
Del Pozo, E., Wyss, H., Tolis, G., Alcaniz, J., Campana, A., and Naftolin, F. (1979). Prolactin and deficient luteal function. Obstet. Gynecol. 53, 282-286
Dixon, W., and Massey, F., Jr. (1969). Introduction to Statistical Analyses, 3rd ed., McGraw Hill, New York.
Dodson, K.S., MacNaughton, M.C., and Coutts, J.R.T. (1975). Infertility in women with apparently ovulatory cycles. Brit. J. Obstet. Gynecol. 82, 615-624
Garcia, C.R. and Rosenfeld, D. (1977). Human Fertility, The Regulation of Reproduction, Davis, Philadelphia.
Jones, G. (1973). Luteal phase insufficiency. Clin. Obstet. Gynecol. 16, 255-273.
Jones, G. (1976). The luteal phase defect. Fertil. Steril. 27, 351-356.
Masnick, G.S., and McFalls, J.A. (1978). Those Perplexing US Fertility Swings. Population Reference Bureau, ISSN 0146-7646
Rosenfeld, D.L, and Garcia, C.R. (1976). A comparison of endometrial histology with simultaneous plasma progesterone determination in infertile women. Fertil. Steril. 27, 1256-1266.
Sherman, B.M. and Korenman, S.G. (1974a). Measurement of serum LH, FSH, Estradiol and progesterone in disorders of the human menstrual cycle: the inadequate luteal phase. J. Clin. Endocrinol. Metab. 39, 145-149.
Sherman, B.M. and Korenman, S.G. (1974b). Measurement of plasma, LH, FSH, estradiol and progesterone in disorders of the human menstrual cycle: the short luteal phase. J. Clin. Endocrinol. Metab. 38, 89.
Taubert, H.D. (1978). Luteal phase insufficiency. Contr. Gynecol. Obstet. 4, 78-113.
Vande Wiele, R.L., Bonumil, J., Dyrenfurth, I., Ferin, M., Jewelewicz, R. Warren, M., Rizkallah, J., and Mikhail, G. (1970). Mechanisms regulating the menstrual cycle in women. Rec. Prog. Horm. Res. 26, 63-95.
Wilks, J. W., Hodgen, G.D., and Ross, G.T. (1976). Luteal phase defects in the rhesus monkey: the significance of serum FSH: LH ratios. J. Clin. Endocrinol. Metab. 43, 1261-1267.
Wilks, J. W., Hodgen, G.D., and Ross, G.T. (1977). Anovulatory menstrual cycles in the rhesus monkey: the significance of serum FSH/LH ratios. Fertil. Steril. 28 (10), 1094-1100.